Pfizer says it has enough promising data on its respiratory syncytial virus, or RSV, vaccine designed to protect newborns that it will end enr…
The US Food and Drug Administration on Wednesday gave emergency use authorization to updated Covid-19 boosters for children as young as 5.
Younger children could soon be eligible to receive an updated Covid-19 booster shot.
The US Food and Drug Administration on Wednesday authorized updated Covid-19 vaccine booster shots from Moderna and Pfizer. This is the first …
Moderna on Friday filed patent infringement lawsuits against Pfizer and BioNTech "for infringing patents central to (its) mRNA technology plat…
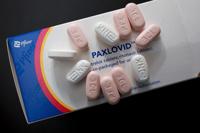
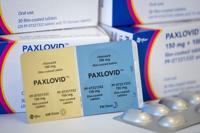
As the coronavirus evolves, the number of treatment options that remain effective against new variants has dwindled. Pfizer's antiviral pill P…
Vaccine maker Pfizer says it has begun Phase 3 clinical trial of its vaccine candidate against Lyme disease with French vaccine company Valneva SE.
Covid-19 vaccines could be authorized for the United States' youngest children as early as June, according to the US Food and Drug Administrat…
To help fend off another wave of Covid-19, people will need a fourth dose of vaccine, Pfizer CEO Albert Bourla told CBS on Sunday.
Pfizer has begun a Phase 2 and 3 clinical trial of its Covid-19 antiviral treatment, Paxlovid, in children ages 6 to 17, the company said Wedn…